Adsorption of linear alkyl benzene sulfonates on oil-water interface: Effects of Na+, Mg2+ and Ca2+ ions
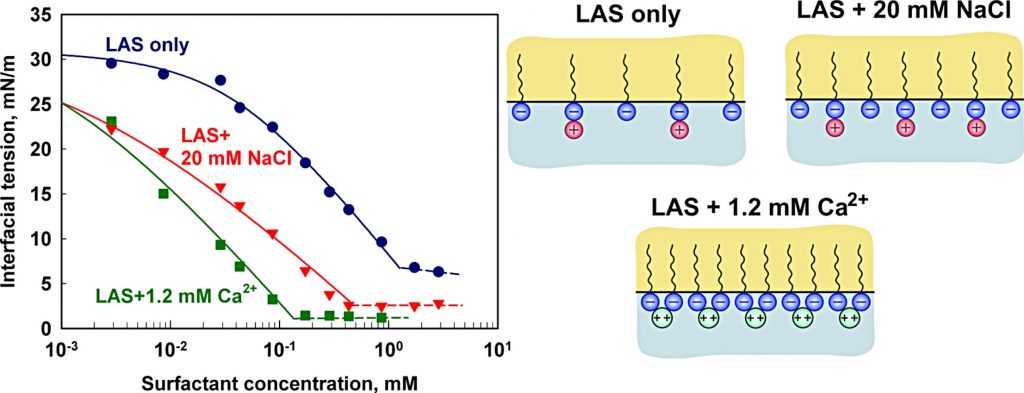
Linear alkyl benzene sulfonates (LAS) are among the most important industrial and house-hold surfactants. Here we study the LAS adsorption properties at oil-water interface in the presence of divalent counter-ions (Mg2+ and Ca2+). Interfacial tension data are obtained and interpreted using a detailed thermodynamic model for surfactant adsorption, which explicitly accounts for counter-ion binding (Kralchevsky et al. Langmuir 15 (1999) 2351). The obtained results show that the hardness ions (i) reduce very significantly the area-per-molecule in the adsorption layer; (ii) reduce strongly the magnitude of the negative surface potential, neutralizing almost completely the adsorbed LAS molecules; (iii) bind strongly to the adsorption layer via both electrostatic attraction and specific attraction of magnitude around 3.5kT. The limiting area-per-molecule at oil-water interface is shown to be significantly larger than the respective area at air-water interface. The latter result indicates that the oil molecules are able to intercalate in between the surfactant molecules in the adsorption layer and, thus, to disrupt the molecular packing at the oil-water interface.

