Synergistic growth of giant wormlike micelles in ternary mixed surfactant solutions: Effect of octanoic acid
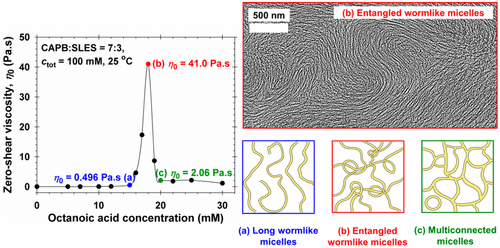
The synergistic growth of giant wormlike micelles in ternary mixed solutions composed of an anionic surfactant (sodium laurylethersulfate, SLES), a zwitterionic surfactant (cocamidopropyl betaine, CAPB), and octanoic acid (HC8) is studied. Rheological data and their analysis in terms of Cole-Cole plots and micellar characteristic times are presented, and the micellar structures behind the observed rheological behavior are revealed by cryo-TEM micrographs. The surfactant composition is fixed near the maximal micelle size of the binary SLES + CAPB system, whereas the concentration of HC8 is varied. At a given HC8 concentration, the viscosity of the ternary micellar solutions exhibits a very high and sharp peak. Polarized-light optical microscopy indicates that all investigated solutions are isotropic rather than liquid-crystalline. The cryo-TEM imaging shows complex phase behavior: wormlike micelles to the left of the peak, giant entangled wormlike micelles at the peak, and long wormlike micelles coexisting with multiconnected micellar aggregates to the right of the peak. The formation of multiconnected micelles leads to a drop in viscosity at the higher concentrations. The results contribute to a better understanding of the structure-rheology relations in micellar surfactant solutions and could be useful for controlling the properties of formulations in personal-care and house-hold detergency.

