Surface properties of adsorption layers formed from triterpenoid and steroid saponins
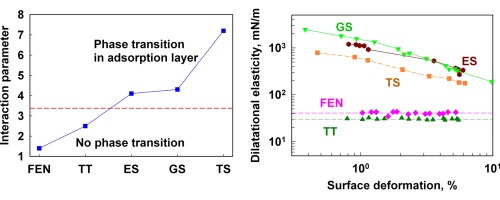
Saponins are natural surfactants with non-trivial surface and aggregation properties which find numerous important applications in several areas (food, pharma, cosmetic and others). In the current paper we study the surface properties of ten saponin extracts, having different molecular structure with respect to the type of their hydrophobic fragment (triterpenoid or steroid aglycone) and the number of sugar chains (1 to 3). We found that the triterpenoid saponins Escin, Tea Saponin and Ginsenosides have area per molecule in the range between 0.5 and 0.7nm2, and the adsorbed molecules are orientated perpendicularly to the interface. The comparison of the experimentally measured surface elasticities with theoretically estimated ones shows that the saponins with very high dilatational and shear elasticities (up to 2000mN/m) have molecular interaction parameter in the adsorption layers which is above the threshold value for two-dimensional phase transition. In other words, the highly elastic layers are in surface condensed state, due to strong attraction between the adsorbed molecules. Furthermore, these adsorption layers have non-linear rheological response upon expansion and contraction, even at relatively small deformation. Layers from the other studied saponins (steroids and crude mixtures of triterpenoid saponins), which are unable to form strong intermolecular bonds within the adsorption layer, have zero shear elasticity and viscosity and low dilatational elasticity and viscosity, comparable in magnitude to those reported in literature for protein adsorption layers. The comparison of the results, obtained by several independent experimental methods, allowed us to formulate the conditions under which the results from different interfacial rheology tests could be compared, despite the complex non-linear response of the saponin adsorption layers.

