Rheology of particle/water/oil three-phase dispersions: Electrostatic vs. capillary bridge forces
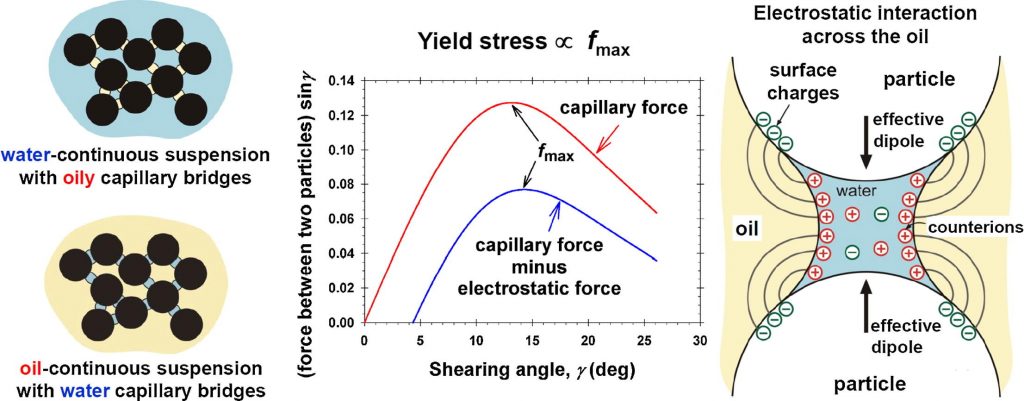
Hypothesis Particle/water/oil three-phase capillary suspensions possess the remarkable property to solidify upon the addition of minimal amount of the second (dispersed) liquid. The hardening of these suspensions is due to capillary bridges, which interconnect the particles (pendular state). Electrostatic repulsion across the oily phase, where Debye screening by electrolyte is missing, could also influence the hardness of these suspensions. Experiments We present data for oil-continuous suspensions with aqueous capillary bridges between hydrophilic SiO2 particles at particle volume fractions 35–45%. The hardness is characterized by the yield stress Y for two different oils: mineral (hexadecane) and vegetable (soybean oil). Findings and modelling The comparison of data for the “mirror” systems of water- and oil-continuous capillary suspensions shows that Y is lower for the oil-continuous ones. The theoretical model of yield stress is upgraded by including a contribution from electrostatic repulsion, which partially counterbalances the capillary-bridge attraction and renders the suspensions softer. The particle charge density determined from data fits is close to that obtained in experiments with monolayers from charged colloid particles at oil/water interfaces. The results could contribute for better understanding, quantitative prediction and control of the mechanical properties of solid/liquid/liquid capillary suspensions.

